William S. Harris, Nathan L. Tintle, […] The Fatty Acids and Outcomes Research Consortium (FORCE)
Abstract
The health effects of omega-3 fatty acids have been controversial. Here we report the results of a de novo pooled analysis conducted with data from 17 prospective cohort studies examining the associations between blood omega-3 fatty acid levels and risk for all-cause mortality. Over a median of 16 years of follow-up, 15,720 deaths occurred among 42,466 individuals. We found that, after multivariable adjustment for relevant risk factors, risk for death from all causes was significantly lower (by 15–18%, at least p < 0.003) in the highest vs the lowest quintile for circulating long chain (20–22 carbon) omega-3 fatty acids (eicosapentaenoic, docosapentaenoic, and docosahexaenoic acids). Similar relationships were seen for death from cardiovascular disease, cancer and other causes. No associations were seen with the 18-carbon omega-3, alpha-linolenic acid. These findings suggest that higher circulating levels of marine n-3 PUFA are associated with a lower risk of premature death.
Introduction
The n-3 polyunsaturated fatty acid (PUFA) family has been the subject of intense investigation ever since their inverse associations with risk for acute myocardial infarction were reported in Greenland Eskimos in the 1970s1,2. The PUFAs in this family include the 18-carbon, plant-derived alpha-linolenic acid (ALA,) as well as the 20–22-carbon, long-chain (LC, mostly seafood-derived) eicosapentaenoic (EPA), docosapentaenoic (DPA), and docosahexaenoic (DHA) acids.
The efficacy of the LC n-3 PUFAs in reducing risk for cardiovascular disease (CVD) remains controversial as findings from different randomized controlled trials (RCTs) have been conflicting. Nevertheless, a 2019 meta-analysis of RCTs reported significant reductions in risk for myocardial infarction, coronary heart disease (CHD) events and mortality, and CVD mortality in patients randomized to supplemental LC n-3 PUFAs3. Another meta-analysis of observational studies found that higher levels of circulating LC n-3 PUFA levels were significantly associated with a lower risk for CHD death4. However, no meta-analysis has yet examined the relationship between LC n-3 PUFAs blood levels and risk for all-cause mortality. Indeed, the only meta-analyses to report a beneficial association with all-cause mortality were based on the self-reported intake of fish5,6. Fish contain many nutrients besides just LC n-3 PUFAs, self-reported food intake is memory dependent, food databases can be out of date, and fish meals often replace less healthful choices. As a result, studies that link LC n-3 PUFAs and health outcomes based on self-reported fish intake have potential limitations. A more reliable and objective measure of LC n-3 PUFA consumption is their level in the blood7 which is primarily determined by the consumption of preformed LC n-3 PUFAs (although synthesis from dietary ALA can make a small contribution8). Hence a clearer picture of the biological relationship between LC n-3 PUFAs and disease outcomes may be obtained from biomarker-based investigations.
Some studies have reported inverse relations between n-3 PUFA biomarkers and total mortality9,10,11, while others have not12,13. In the Cardiovascular Health Study, higher LC n-3 PUFA levels also were associated with overall “healthier aging” (i.e., surviving past age 65 free of chronic diseases and maintaining good functional status)14. However, reports from studies of individual cohorts can be limited by insufficient power and inconsistent adjustment for potential confounding factors. In addition, publication bias can distort summary conclusions. To address these challenges, the present study pooled de novo individual-level analyses across 17 prospective cohort studies in the Fatty Acid and Outcome Research Consortium (FORCE)15 to explore the associations of circulating levels of n-3 PUFAs (both plant- and seafood-derived) and all-cause mortality. Secondarily, we examined the associations with mortality from CVD, cancer, and all other causes.
Here, we show significant inverse associations for all mortality endpoints with the LC n-3 PUFA levels. Hence, chronically higher tissue levels of these FAs operating through a variety of potential mechanisms may slow the aging process.
Results
Population
The pooled analyses included circulating n-3 PUFA measurements on 42,466 individuals, 15,720 (37%) of whom died during follow-up (Table 1). At baseline, the average age was 65 years (range of mean ages across cohorts was 50–81 years), 55% were women (range of 0–100% across cohorts) and the median follow-up time was 16 years (range of 5–32 years across cohorts). Whites constituted 87% of the sample. Circulating levels of the n-3 PUFAs (and of the n-6 PUFAs linoleic and arachidonic acids, which were included as covariates) are shown in Supplementary Fig. 1 and in Supplementary Table 2. Supplementary Table 3 shows the number of cause-specific deaths from participating cohorts. Overall, approximately 30% of the deaths were attributed to CVD, 30% to cancer, and the remaining 39% to all other causes.
Table 1 Baseline characteristicsa of 17 prospective cohort studies included in the meta-analysis: Fatty Acids and Outcomes Research Consortium.
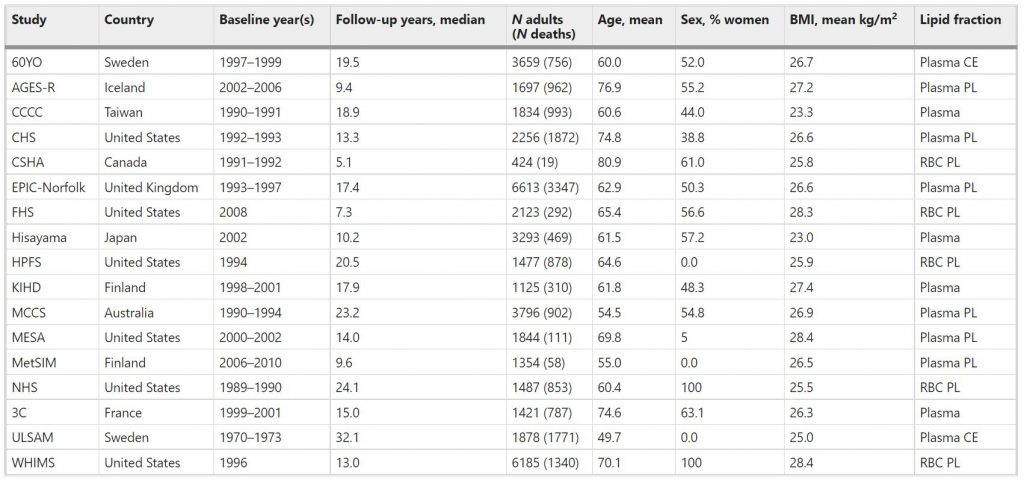
Abbreviations of cohorts: 60YO, Stockholm cohort of 60-year olds, AGES-R Age, Genes, Environment Susceptibility Study (Reykjavik), CCCC Chin-Shan Community Cardiovascular Cohort Study, CHS Cardiovascular Health Study, CSHA Canadian Study of Health and Aging, EPIC-Norfolk European Prospective Investigation into Cancer, Norfolk UK, FHS Framingham Heart Study, HPFS Health Professionals Follow-up Study, KIHD Kuopio Ischemic Heart Disease Risk Factor Study, MCCS Melbourne Collaborative Cohort Study, MESA Multi-Ethnic Study of Atherosclerosis, MetSIM Metabolic Syndrome in Men Study, NHS Nurses’ Health Study, 3C Three-City Study, ULSAM Uppsala Longitudinal Study of Adult Men, WHIMS Women’s Health Initiative Memory Study. CE cholesteryl esters, PL phospholipids, RBC red blood cells.
Total mortality
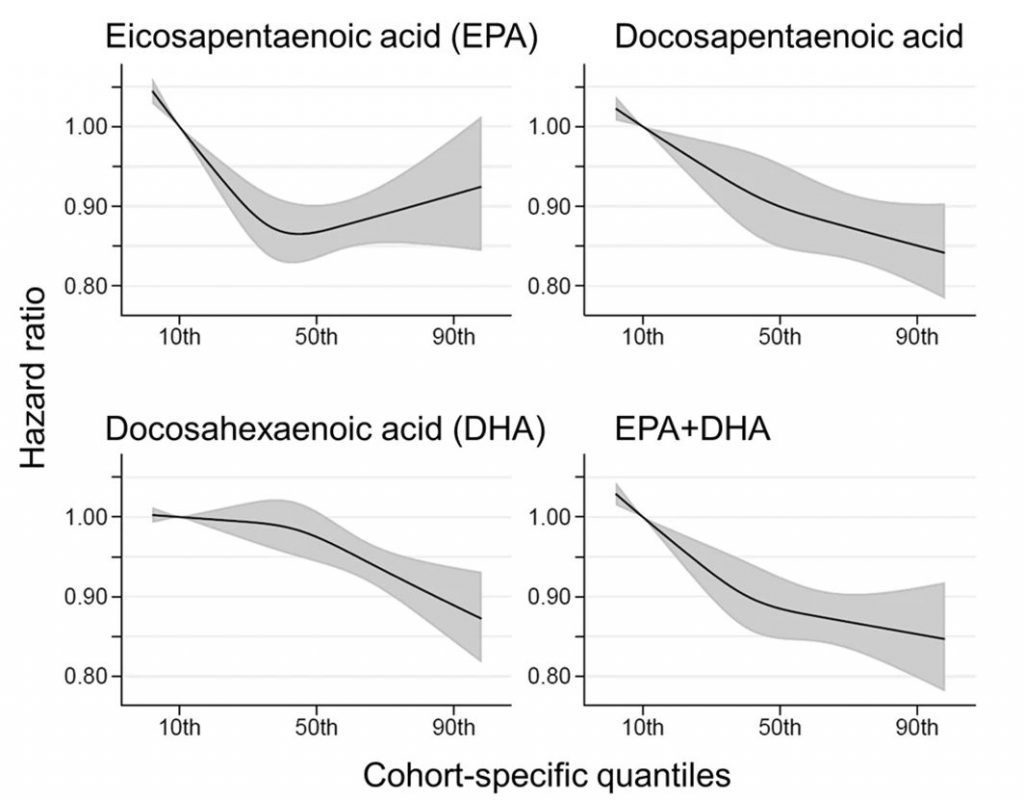
Comparing the medians of the first and fifth quintiles (i.e., approximately the 90th and the 10th percentiles), higher EPA, DPA, DHA, and EPA + DHA levels were associated with between 9% and 13% lower risk of all-cause mortality (Table 2). (The fatty acid levels associated with these percentiles for each cohort and sample type are shown in Supplementary Table 4). The HR for total mortality for EPA + DHA was 0.87 (95% CI: 0.83–0.90) (Fig. 1). In contrast, ALA was not significantly associated with all-cause mortality [HR 0.99 (0.96–1.02)]. In an across quintiles analysis, significant trends were observed for EPA, DPA, DHA, and EPA + DHA (all < 0.01); and comparing the top to the bottom quintile, each was associated with 15–18% lower risk of death (Table 3). There was little evidence for nonlinearity in these inverse associations for all each LC n-3 PUFAs except for EPA (p = 0.002 for the nonlinearity; Fig. 2). The relationship of EPA with mortality was most pronounced at lower levels and then appeared to plateau at higher levels. ALA was generally unassociated with total mortality, except for a borderline association in the top quintile [HR 0.94 (0.89–0.99); P-trend = 0.13], and there was no evidence for nonlinearity (Supplementary Fig. 2).

Fig. 1: Adjusted hazard ratios (HR, 95% CI) for total mortality for circulating eicosapentaenoic (EPA) plus docosahexaenoic acid (DHA) in the 17 contributing studies of the Fatty Acids and Outcomes Research Consortium.
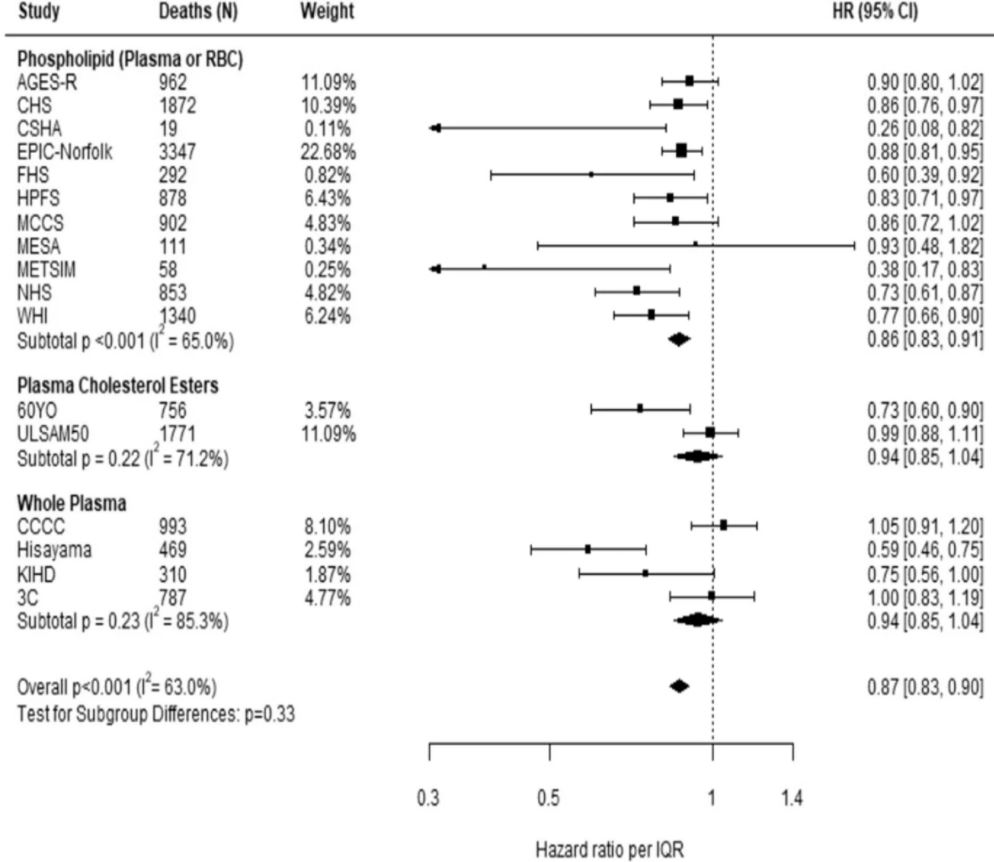
Table 3 Meta-analysis of circulating n-3 PUFA biomarkers with mortality types by cohort-specific quintiles (hazard ratios and 95% CIsa): Fatty Acids and Outcomes Research Consortium.
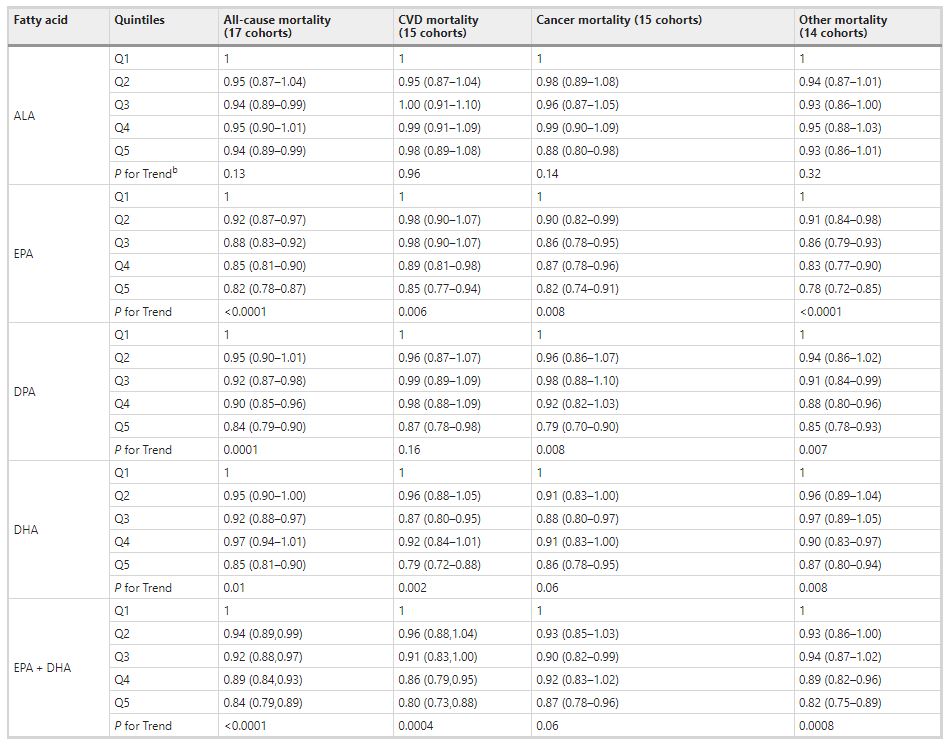
bP-for trend is computed by using a fixed-effects, inverse weighted meta-regression analysis, i.e., the hazard estimates were regressed against study quintiles, which we assigned a value of 1, 2, 3, 4, or 5.
Abbreviations: ALA alpha-linolenic acid, CI confidence interval, CVD cardiovascular disease, DHA docosahexaenoic acid, DPA docosapentaenoic acid, EPA eicosapentaenoic acid.
Fig. 2: Associations of circulating long-chain n-3 PUFA levels with all-cause mortality: nonlinear dose-response meta-analysis in the Fatty Acids and Outcomes Research Consortium. Hazard ratios and cohort-specific quantiles are presented in the vertical and horizontal axis, respectively.
Cause-specific mortality
Comparing the 90th to the 10th percentile, each of the LCn-3 PUFAs was significantly associated with a lower risk for death from CVD, cancer, and all other causes combined [except for DHA and cancer mortality, HR 0.93 (0.86–1.00)] (Table 2). ALA was not significantly associated with any cause-specific mortality. Evaluating the trend across quintiles, EPA, DHA, and EPA + DHA were inversely associated with CVD death, EPA and DPA were inversely associated with cancer death, and each of the LC n-3 PUFAs was inversely associated with other death. Comparing the top to the bottom quintile, EPA, DPA, DHA, and EPA + DHA were each significantly, inversely associated with CVD, cancer, and other mortality (Table 3).
Heterogeneity and sensitivity analyses
Inter-cohort heterogeneity was at least moderate (I2 > 50%) in the pooled analyses of all-cause mortality for all n-3 PUFAs except ALA (I2 = 26%) and EPA (I2 = 41%), while heterogeneity for cause-specific mortality ranged from little to moderate (0–56%) (Supplementary Table 5). There was little evidence of differential associations with mortality by PUFA lipid compartment after accounting for multiple testing (5 PUFAs × 4 outcomes; Bonferroni correction 0.05/20 = 0.0025, Supplementary Table 6). Likewise, associations of n-3 PUFAs with total mortality were similar across strata based on age, sex, race, and fish oil use (Supplementary Table 7), with no significant differences after accounting for multiple testing (5 PUFAs × 4 strata results; Bonferroni correction 0.05/20 = 0.0025). Overall findings did not change with the removal of participants taking fish oil (Supplementary Table 7) or in the drop-one-cohort analyses.
Discussion
In this meta-analysis utilizing a harmonized analytical strategy with individual-level data from 17 cohorts, we examined the associations between circulating levels of the n-3 PUFAs and mortality. We found that, after controlling for other major risk factors, LC n-3 PUFAs (but not ALA) were associated with about a 15–18% lower risk of total mortality comparing the top to the bottom quintiles. These relationships were generally linear for DPA, DHA, and EPA + DHA, but not for EPA. For this PUFA there was a steeper risk reduction across the lower blood levels but little additional difference in risk at higher blood levels. Inverse correlations were also generally observed between LC n-3 PUFA levels and CVD, cancer, and other causes of death.
This pooled analysis including over 40,000 participants and over 15,000 deaths greatly expands upon the findings of prior individual cohort studies that examined associations of circulating levels of n-3 PUFAs and all-cause mortality9,10,11,12,13,16,17,18,19,20,21,22,23,24. Relatively few studies have evaluated self-reported dietary fish (or estimated n-3 PUFA) intake in relation to total mortality, but those that have typically support our observations here5,22,25,26. Interestingly, reported use of fish oil supplements was linked to a lower risk for death from any cause in a study from the UK including over 427,000 individuals27.
Associations with total and cause-specific mortality were not significant for the plant-derived n-3 PUFA ALA. Prior biomarker-based meta-analyses reported inverse associations of ALA with CHD death, but relationships with total or CVD mortality were not examined4,28. Whether our finding of no association ALA on CVD mortality was because ALA has no role to play in fatal strokes (included in the CVD mortality metric) or because of differences in the cohorts included in these prior meta-analyses vs. the present one is not clear. Circulating ALA levels are less dependable markers of intake compared with the LC n-3 PUFAs because this fatty acid is rapidly β-oxidized and, to a small extent, converted into the LC n-3 PUFAs8. Nevertheless, the borderline and inconsistent relations of ALA on mortality risk deserve further study.
Higher circulating levels of LC n-3 PUFAs may beneficially affect diverse cellular systems that together could contribute to a reduced risk for death. The mechanisms behind the ostensibly beneficial effect of LC n-3 PUFAs on human biology are multiple and have been summarized in several recent reviews papers29,30,31,32. Among them are hypotriglyceridemic, antihypertensive, and antiplatelet effects; as well as positive effects on adipocyte biology, endothelial function, and autonomic balance. All of these appear to be mediated by effects on membrane physiochemistry, gene expression, and the production of a myriad of bioactive oxylipins. Persistently lower levels of inflammatory biomarkers also characterize those with higher circulating LC n-3 PUFA levels33. These fatty acids have been reported to inhibit the mammalian (or mechanistic) target of rapamycin (mTOR) in animal studies showing benefits in cancer34, metabolic syndrome35, spinal cord injury36, and depression37. mTOR inhibition extends lifespan in many species38 and acts as an energy sensor to coordinate gene expression, ribosome biogenesis, and mitochondrial metabolism39. In the Heart and Soul Study, where whole blood EPA + DHA levels were inversely associated with all-cause mortality24, higher levels were also linked with a slower rate of telomere shortening over a 5-year period40. As higher rates of telomere attrition have been associated with shorter overall lifespan41,42, this finding may be secondary to the more distal biochemical mechanisms noted above. Regardless of their specific actions, higher cellular levels of the LC n-3 PUFAs appear to slow the aging process.
Our findings of lower risk of CVD death with high vs. low blood levels of EPA + DHA are generally consistent with meta-analyses of self-reported fish intake25 and of biomarker levels4, as well as randomized controlled clinical trials of n-3 PUFA supplementation3,43 (although the most recent trial44 has not yet been included in meta-analyses). Compared with CVD, evidence for a link between n-3 PUFAs and cancer mortality risk is sparse, with no significant relationship for self-reported estimates of fish or n-3 PUFA consumption25,45. Meta-analyses of RCTs with n-3 PUFA supplements also have not observed effects on cancer, although short-term durations of such trials (generally up to 5 years) would likely preclude any ability to detect an effect on cancer46,47. The difference between these findings and what we observed may arise from the use of biomarker levels instead of self-reported fish intake. Biomarkers are potentially truer reflections of long-term exposure, making it easier to detect subtle relationships. In addition, circulating LC n-3 PUFA levels reflect endogenous metabolism, especially for DPA which is not correlated with estimated dietary DPA intake48 but may have important biologic effects49. Finally, since neurodegenerative diseases are a major non-CVD, non-cancer cause of death, a report that higher fish intake was associated with reduced mortality from this cause6 is consistent with our observations here.
Although circulating marine n-3 PUFA levels have not been measured in all of the major intervention trials, the doses of EPA + DHA used in most trials (<1 g/day) may not have resulted in marked differences in levels between treated and control patients50. For example, in the Vitamin D and Omega-3 Trial (VITAL) trial, treatment with 840 mg of EPA + DHA per day increased plasma phospholipid EPA + DHA levels from 2.7 to 4.1%, a 55% increase. This relatively small difference in LC n-3 PUFA levels between the placebo and active treatment groups could be one of the potential reasons for the failure of some RCTs to detect an effect of n-3 PUFAs on CV outcomes50,51. Future RCTs may be more effective if they focus on people with low baseline levels of LC n-3 PUFAs52 and provide doses of EPA and DHA that produce higher blood levels. An intake of about 250 mg of EPA + DHA per day as recommended in the Dietary Guidelines for Americans53 may raise circulating levels into the ranges observed here for some but not all adults7.
Although a significant effect on the primary (composite) endpoint in the VITAL trial47 was not achieved, our findings comport well with some of its secondary findings. In this study, the provision of 840 mg of EPA + DHA/day significantly reduced risk for major CV events and myocardial infarction in those participants with lower (vs. higher) intakes of fish (blood levels in these groups were not reported). There was a significant interaction of fish intake on total mortality as well; the HR (95% CI) in the low intake group was 0.87 (0.73–1.04) and in the high intake group, 1.19 (0.99–1.44, p for interaction 0.017). This secondary observation in VITAL implies that individuals with lower baseline LC n-3 PUFA levels are more likely to benefit from increased levels than those with higher baseline levels. Two recent RCTs examining the effects of high dose (~3–4 g/day) of LC n-3 PUFAs were performed in overweight patients with high blood triglyceride levels and at high risk for CVD events, all on background statin therapy. After 5 years of treatment, Bhatt et al.54 reported beneficial effects of EPA ethyl esters on CV events, whereas Nicholls et al.44 found no effect on the primary outcome using an EPA + DHA product in which the fatty acids were non-esterified. Another 2-year trial in elderly post-MI patients from Norway given 1.8 g of EPA + DHA found no benefit on CV outcomes55. None of these trials is directly relevant to our findings here owing to the nature of the high-risk patient populations, the number of concurrent background medications, the short duration of treatment, and the initiation of treatment late in life.
Strengths of the current analysis include the use of objective n-3 PUFA biomarkers (instead of estimated intakes from dietary questionnaires) which increases the accuracy of exposure assessment and allows for separate analysis of different individual n-3 PUFAs. The use of prespecified, harmonized, de novo individual-level analyses across multiple cohorts substantially increase generalizability, reduces confounding through consistent adjustment for covariates, and limits the potential for publication bias. The pooling of 17 studies including over 15,000 deaths also increased the statistical power to evaluate mortality subtypes as well as potential heterogeneity across subgroups.
Potential limitations deserve attention. Because our outcome was not rare, the hazard ratios (HRs) reported here (instantaneous relative risk) may be modestly different than the cumulative relative risk. Most individuals were White, potentially lowering generalizability to other races/ethnicities, although our analysis still included nearly 6000 non-Whites in whom findings for EPA + DHA were generally similar to those for Whites (Supplementary Table 7). Despite extensive efforts to harmonize study-specific methods, moderate heterogeneity remained between studies that may be due to unmeasured background population characteristics, differences in laboratory assessment of PUFAs and of outcomes, chance, or any combination of these. PUFAs and covariates were measured once at baseline, and changes over time could lead to misclassification, which could bias the results in uncertain directions. On the other hand, reasonable reproducibility has been reported for n-3 PUFA biomarker concentrations over time56. Because analytical methods, even within the same lipid fraction, were not standardized, and n-3 PUFA levels were measured in multiple fractions, we assessed cohort-specific n-3 PUFA percentiles rather than absolute percentages of total fatty acids in each fraction. Since FA levels were reported as a percent of total FAs in each lipid compartment, levels of one FA could affect levels of another. Indeed, in the plasma or RBC PL and CE pools, higher levels of the LC n-3 PUFAs (which were the focus of this study) are linked with lower levels of the n-6 PUFAs but not of saturated or mono-unsaturated FAs57,58. Since we adjusted for differences in linoleic and arachidonic levels in our analyses, this concern was accounted for. Each lipid pool used in this study reflects LC n-3 PUFA intake during relatively different and overlapping time periods generally from months to weeks following this hierarchy: RBC ≥ Plasma PL ≈ Plasma CE ≥ total plasma59,60. In addition, we cannot rule out the potential for residual confounding. That is, higher LC n-3 PUFA levels may simply be markers of a “healthy lifestyle,” and the fatty acids themselves may not be playing any physiological role in postponing death but would be biomarkers of a suite of other healthy behaviors (dietary/exercise/non-smoking, etc.), or endogenous metabolic processes, that might, in a multiplicity of ways, manifest in greater longevity. Although we adjusted for many major risk factors (age, income, marital status, smoking, hyperlipidemia, hypertension, etc.), residual confounding by other factors is always possible. However, the magnitude of the observed effect of the meta-analysis of circulating LC n-3 PUFAs and total mortality reported herein is consistent with the known associations with CHD mortality and sudden cardiac death61,62. Finally, as the attribution of cause of death is never as unambiguous as death itself, some uncertainty must attend to the cause-specific analyses reported here. In summary, in a global pooled analysis of prospective studies, LC n-3 PUFA levels were inversely associated with risk for death from all causes and from CVD, cancer, and other causes.
https://www.nature.com/articles/s41467-021-22370-2
ChooseLife Notes : None of these studies, on ALA, will have included 37g odd emulsified 18-chain ALA daily, as Budwig studied and found to be the chief propellant to regain electron conductance within the cells. So whilst I like these studies they are biased towards Murine sources unjustly IMO, Flax has the highest level of surplus Electrons, or Pi Electrons, most enriching to our Electron Cloud, as Budwig variously described.
